

Lewis in 1916 with the identification of the chemical bond present in the organic compounds as a pair of electrons was held jointly by two atoms which were holding them together. The first great step in the development of a satisfactory explanation of valence and chemical combination was made by the American chemist G. It later was quite evident, however, that the valencies of many of the elements varied in different compounds. The characteristic valence of elements was measured in terms of the number of atoms of hydrogen with which an atom of the element can merge or that it can replace in a compound. As there was no satisfactory theory for the cause of valency, most of the efforts were done on making empirical rules for the determination of the valencies of the elements. The explanation and the systematization of the concept of valence or valency was one of the major challenges for 19 th -century chemists.
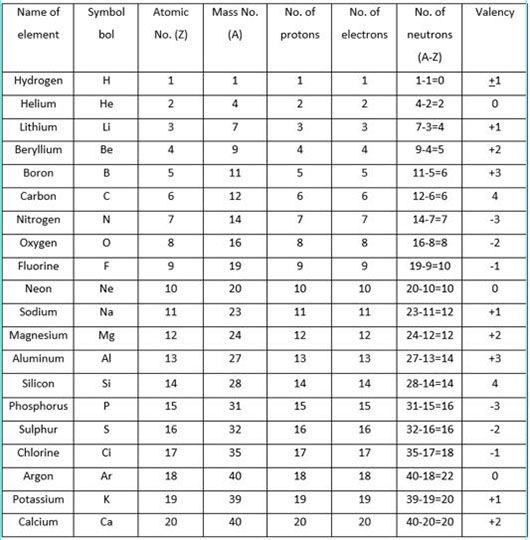
Valency also helps us predict the properties of new compounds that we create. Valency is important because it gives us information about how atoms combine and why certain chemicals react the way they do. The valency of an element can be increased either by gaining or losing electrons. The valency of an atom is determined by the number of electrons in its outermost shell. Valency is an important measure of the combining power of atoms. Valency is a fundamental concept that helps us understand the world around us. This makes it easier to create new compounds for use in various industries. By understanding valency, we can better predict how chemicals will react with each other. Valency is also an important concept in chemistry because it helps us understand how atoms join together to form molecules. This is why elements with a high valency are often used in chemical reactions - they form strong bonds with other atoms. The higher the valency, the stronger the bond. The valency of an element is important because it determines how strong the bond between the atoms will be. The valence electrons determine the valency meaning in Chemistry and define what is valences in Chemistry. Thus valence meaning is the number of electrons as most of the bonds are formed by the exchange of the valence electrons. Introduced in 1868, the term is used for the expression of both the possibility of combination of an element in general and the numerical value of the power of combination. The Valence, or known as valency, in Chemistry, is the characteristics of an element that indicates the number of other atoms with which an atom of a particular element can form a covalent bond.
:max_bytes(150000):strip_icc()/PeriodicTableValence-58b5d8f95f9b586046df59fb.jpg)
The valency of an element is determined by the number of electrons in its outermost shell. Valency is a measure of the combining power of an atom. Valency is also known as molecular weight. Valency is the number of atoms of a particular element that is combined with one atom of another element to form a molecule.


 0 kommentar(er)
0 kommentar(er)
